

Infant incubators and radiant warmers are medical devices that aim to provide safe and stable environments for newborn infants to heal, grow, and develop. When babies are born, they are often placed under radiant warmers to stabilize their temperature until they can achieve self-thermoregulation. While welcoming a new life into the world is a wonderful and awe-inspiring event, sometimes things don’t always go as planned. Many babies are born with illnesses, disabilities, or prematurely.
According to the World Health Organization, approximately 15 million babies are born premature each year worldwide. These small patients are especially vulnerable for the first several days—or even months—of life. This is when the use of infant incubators becomes essential. Incubators are controlled enclosures that are designed to replicate the environment of the mother’s womb as much as possible by modulating temperature, humidity, and airflow, minimizing sound, and often, providing an oxygen-controlled environment.
Fully enclosed, an infant incubator carefully controls its environment to protect infants during their earliest stages of life when they’re most vulnerable. The incubator may include an AC-powered heater, a water container to add humidity, a motorized fan to circulate the warm and humid air throughout the cabin of the incubator, a control valve through which oxygen may be added, and a servo-control to help regulate air temperature (a temperature sensing thermistor taped to the infant’s abdomen).
Hand-access ports with doors limit the introduction of cooler air while the infant is being handled, and the hood or side panel can be opened to gain greater access to the infant. The heat circulated in the cabin is then absorbed into the body by blood convection and tissue conduction, ideally keeping both the skin and core temperature maintained by minor variations.
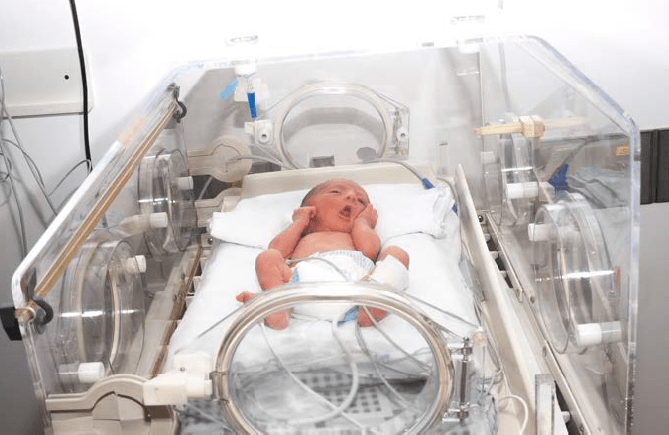
Example of infant incubator
Radiant warmers are regularly used in delivery rooms and neonatal care units to simultaneously provide external heat and open access to newborns. Immediately following birth, infants are routinely placed under the warm, radiant light to help stabilize their temperature until they can achieve self-thermoregulation. Monitoring and resuscitation can easily be performed from the open-access of a radiant warmer, along with any necessary procedures. Radiant warmers are also used for critically ill patients that require constant nursing intervention.
Radiant warmers are usually overhead heating units, consisting of a heat source, skin-temperature sensor, servo-control unit, and both visual and audible alarms. The heating element generates radiant energy in the far IR wavelength region, but is limited to prevent thermal damage to the infant. The IR energy is readily absorbed by the infant’s fragile skin—increasing blood flow in the skin, then transferring the heat to the rest of the infant’s body by blood convection and tissue conduction.
Since a radiant warmer is open to the air, evaporation is a major factor of heat loss. Most radiant warmers have short walls around the perimeter of the mattress to reduce the amount of airflow over the patient and thus limit evaporative heat loss.
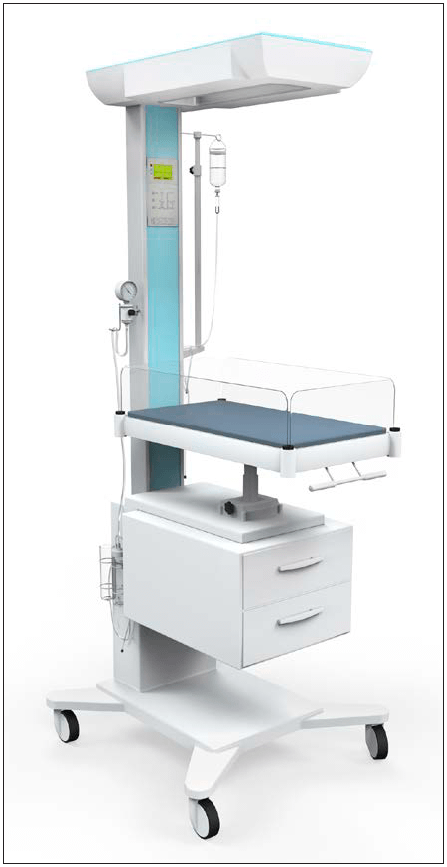
Example of radiant warmer
Standards may be international or local national (by country). The IEC standards reflect the best experience of the industry, researchers, consumers, and regulators worldwide. They help establish a uniform and universal testing procedure for biomedical, service, and design engineers to use, and are critical in helping properly assess the safety of medical devices and ensuring patient safety. For this reason, most of the local national (by country) standards have been harmonized to the IEC standards, which are recognized as the international preferred practice. The IEC 60601-2-19, 60601-2-20, and 60601-2-21 standards recommend basic safety and essential performance requirements for infant incubator, transport incubator, and radiant warmer testing.
While voluntary, most manufacturers recognize that the customers in the markets they service will hold them accountable to these standards and will thus base their testing procedures around them. A manufacturer’s service manual will contain procedures specific to the maintenance and performance of the device, and typically recommend an inspection frequency. While these testing workflows tend to not to be quite as thorough as the standards, each manufacturer applies the standards to the best testing for the design of their brand and model of infant incubator, transport incubator, or radiant warmer.
The manufacturer’s manual will give model-specific directions to test the incubator or radiant warmer, such as the need to ensure warning lights turn on when they should (i.e. if the temperature is too high, the alarm should sound). Complete the performance inspection per the standards and/or manufacturer’s procedures.
An infant’s first few days of life can be their most critical, which is why it’s important to routinely test and verify the safety and performance of infant incubators and radiant warmers. Check the manufacturer’s service manual for frequency recommendations; most manufacturers recommend a minimum inspection frequency of once per year.
If the service manual and inspection procedure from the manufacturer is not available, the frequency of inspection must still be determined. One method for determining how often a medical device should be tested is a risk-based method used by the University of Vermont Biomedical Engineering Department. As shown in the table below, this method is described in Medical Equipment Quality Assurance: Inspection Program and Development Procedures by J. Tobey Clark.
| Criteria - choose 1 rating from each category | Weight | Score |
| Clinical function | ||
| No patient contact | 1 | |
| Device may make contact with patient but function is non-critical | 2 | |
| Device is used for patient diagnosis, or direct monitoring | 3 | |
| Device is used to deliver direct treatment to the patient | 4 | 4 |
| Device is used for a life support | 5 | |
| Physical risk | ||
| Device poses no appreciable risk due to failure | 1 | |
| Device failure will result in low risk | 2 | |
| Device failure will result in inappropriate therapy, misdiagnosis, or loss of monitoring | 3 | |
| Device failure could result in severe injury to, or death of, patient or user | 4 | 4 |
| Problem avoidance probability | ||
| Maintenance or inspection would not impact reliability of the device | 1 | |
| Common device failure modes are unpredictable or not very predictable | 2 | 2 |
| While common device failure modes are not very predictable, device history indicates that TSP testing frequently detects problems | 3 | |
| Common device failure is predictable and can be avoided by preventive maintenance | 4 | |
| Specific regulatory or manufacturers requirements dictate preventive maintenance or testing | 5 | |
| Incident history | ||
| No significant history | 1 | 1 |
| A significant history of incidents exist | 2 | |
| Manufacturers/regulatory requirements for specific schedules | ||
| No requirements | 1 | 1 |
| There are requirements for testing independent of a numerical rating system | 2 | |
| Total Score: | 12 | |
| Assignment: 0.0x 0.5x 1x 2x 3x 4x (times per year tested) | 1 | |
| A combined score of 13 or more is justification for semiannual testing. A combined score of 9-12 is justification for annual testing. A combined score of 8 or less is justification for less than annual testing. | ||
| Criteria - choose 1 rating from each category | Weight | Score |
| Clinical function | ||
| No patient contact | 1 | |
| Device may make contact with patient but function is non-critical | 2 | |
| Device is used for patient diagnosis, or direct monitoring | 3 | |
| Device is used to deliver direct treatment to the patient | 4 | 4 |
| Device is used for a life support | 5 | |
| Physical risk | ||
| Device poses no appreciable risk due to failure | 1 | |
| Device failure will result in low risk | 2 | |
| Device failure will result in inappropriate therapy, misdiagnosis, or loss of monitoring | 3 | |
| Device failure could result in severe injury to, or death of, patient or user | 4 | 4 |
| Problem avoidance probability | ||
| Maintenance or inspection would not impact reliability of the device | 1 | |
| Common device failure modes are unpredictable or not very predictable | 2 | 2 |
| While common device failure modes are not very predictable, device history indicates that TSP testing frequently detects problems | 3 | |
| Common device failure is predictable and can be avoided by preventive maintenance | 4 | |
| Specific regulatory or manufacturers requirements dictate preventive maintenance or testing | 5 | 5 |
| Incident history | ||
| No significant history | 1 | |
| A significant history of incidents exist | 2 | 2 |
| Manufacturers/regulatory requirements for specific schedules | ||
| No requirements | 1 | 1 |
| There are requirements for testing independent of a numerical rating system | 2 | |
| Total Score: | 16 | |
| Assignment: 0.0x 0.5x 1x 2x 3x 4x (times per year tested) | 2 | |
| The risk assessment from Fluke Biomedical differs slightly from the University of Vermont in two categories: Problem avoidance probability and Incident History. Problem avoidance probability was rated a 5 as opposed to 2 because both regulatory and manufacturer guidelines recommend weekly, quarterly, and yearly preventative maintenance schedules. Incident history was rated a 2 instead of 1, as incubator incidences would result in patient harm (if incidences would not result in patient harm, the score would be 1). The total score is 16 (instead of UV’s score of 12), indicating semi-annual (twice per year) testing for infant incubators. | ||
The University of Vermont recommends an annual testing frequency for both incubators and radiant warmers (Figure 1a). However, a semiannual (2 times per year) testing frequency (Figure 1b) for incubators, and annual testing for radiant warmers can be appropriately justified. Whichever frequency you deem is most appropriate for your equipment, facility, and patients, be sure to have a written statement with your justification documented. This statement of policy will come in handy during audits of your quality assurance program.
If the service manual and inspection procedure from the manufacturer is not available, it is still the responsibility of the medical facility to choose and standardize on a test procedure. It is important that the infant incubator and radiant warmer functionality be quantitatively evaluated by comparing it to the applicable medical device standard or manufacturer’s specifications.
If the manufacturer’s specifications are not known, the IEC 60601-2-19, 60601-2-20, and 60601-2-21 standards are a great place to start, and a more than reasonable substitute. The University of Vermont Biomedical Engineering Department has proposed a medical device category-specific inspection work-flow shown in the Medical Device Quality Assurance Program Development and Procedures Book—there is one for infant incubators and another for radiant warmers. Once the inspection criteria have been agreed upon, no changes should be made without a rationale statement describing why a change was required, what the change was, and how this change was validated.
Probe placement when testing parameters—especially temperature and airflow—are common mistakes when testing and performing preventative maintenance on infant incubators.
Sensors for all parameters, including temperature, humidity, airflow, and sound should be ten centimeters above the mattress (Figure 2)—which is approximately the height an infant would comprise when lying flat on the mattress in the incubator.
Temperature (air convection temperature) and airflow should be measured in five locations inside the incubator. Capturing the temperature in a single location is not acceptable. This makes sense, as an infant’s torso and limbs may be stretched across more than the very center of the mattress. Thus, we need to ensure the environmental conditions are uniform across the entire chamber. Maintaining an infant’s core and skin temperature is absolutely critical. As we think back to how an incubator functions, a motorized fan circulates heated air throughout the chamber of the incubator, and heat loss from the infant by convection is dependent upon air speed and air temperature. Measuring both the temperature and airflow of the incubator in more than one location will give good insight into the actual performance of the medical device, ensuring the uniformity of environmental parameters.
If we were to divide the mattress into four equal quadrants (see Figure 2), temperature and airflow probes should be placed in the very center of the mattress (M), and in the center of each quadrant (A, B, C, D). Remaining parameters should be measured from the central point of the mattress.
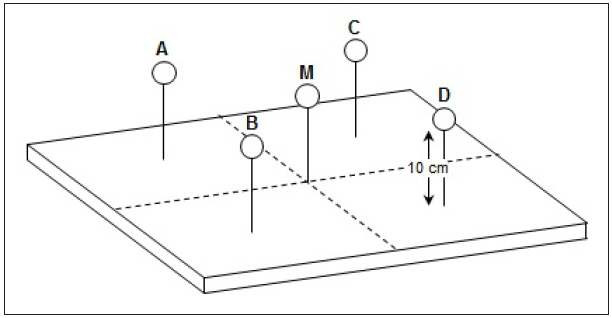
Figure 2. Air temperature probe height and placement on mattress
NOTE: The pattern of airflow differs in every model of incubator, so it is important to be familiar with the direction of airflow inside the incubator that is being tested, and place the airflow probe properly to obtain an accurate measurement. For example, a bi- or uni-directional airflow probe needs to be placed perpendicular to the direction of airflow to obtain an accurate reading. Measurements should be taken 10 cm from above the mattress, in the center of the mattress, and the center of each quadrant.
To measure the radiant heat from a radiant warmer, ensure an appropriate sensor is being used to capture accurate readings. For example, the Fluke Biomedical INCU II Incubator/Radiant Warmer Analyzer uses aluminum pucks to absorb and accurately measure the heat produced from the warmer. Again, the temperature probes should be placed in five locations—center of the mattress, and center of each quadrant as shown in Figure 2—and readings subsequently recorded.
Warm-up time is the time it takes for the incubator to rise 11 °C from ambient temperature. Manufacturers’ manuals will specify the warm-up time, and testing should be carried out to ensure the incubator is within the acceptable range of ±20 % the specified warm up time. This is very important, as incubators are often turned on right before use.
If the manufacturer specifies their incubator takes 20 minutes to warm-up, a neonatal nurse will expect the incubator to be at the appropriate temperature 20 minutes after the incubator has been turned on. Since newborns and critical care infants are extremely sensitive to environmental changes, it is imperative incubators be in proper working condition and warmed to a stable temperature when an infant is placed inside.
Once an incubator has warmed to its set temperature, the incubator will overshoot the set point. This overshoot should be no more than 2 ºC above the set point. After the overshoot, the temperature will then undershoot and overshoot in minor variations, oscillating to an average, steady incubator temperature. STC—steady temperature condition—is a condition that is reached when the incubator temperature does not vary by more than 1 ºC over a period of one hour (Figure 3).
Simultaneously measuring each parameter both during warm-up time and after STC helps give the end user an insight into how the device is running as a whole, and where problems might be occurring if the incubator is not functioning properly. Keep in mind that each time a porthole or cabin door is opened, STC is lost and must be achieved again.
Most manufacturer performance inspection procedures require electrical safety tests, including ground wire resistance and chassis leakage. Keep an electrical safety analyzer close by to complete the electrical safety portion of the performance inspection easily. While some treat the electrical safety and other integrated device testing as separate work-flows, they are generally treated by the manufacturer and the standards as a single series of work and measurements with one overall test result file and report.
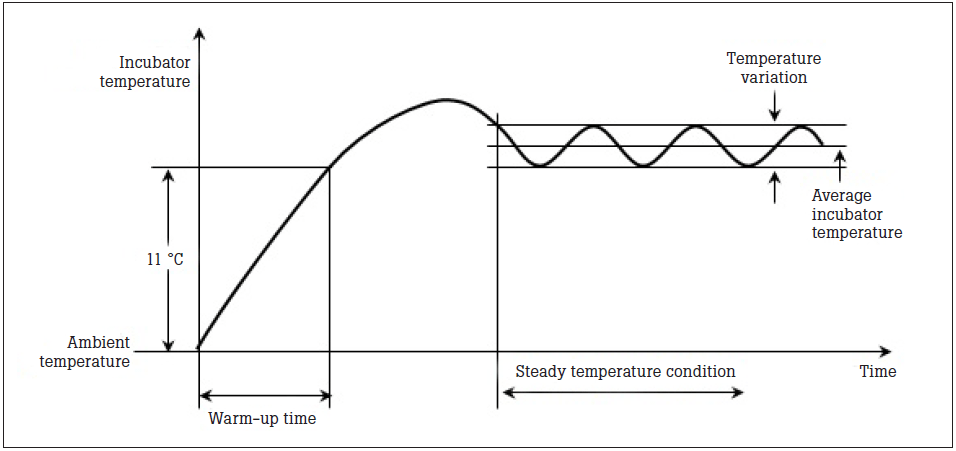
Figure 3. Variation of Incubator Temperature.

ESA 612 Electrical Safety Analyzer

MaxO2 Oxygen Analyzer

ProSim 4 Vital Signs Patient Simulator

SPOT Light Pulse Oximeter Analyzer
Along with providing an oxygen-controlled environment, many incubators and radiant warmers also contain built-in SpO2 and monitoring systems, and are part of the testing and preventative maintenance for the incubator/radiant warmer.
A complete list of tests and criteria can be found in the IEC 60601-2-19 and 60601-2-21 standards for infant incubator and radiant warmer testing, or refer to the manufacturer’s service manual. Here are the basics:
Temperature: Make sure the incubator warms up in the time specified in the manufacturer’s manual. Test to ensure the overshoot temperature does not exceed 2 °C of the set point. Check all surfaces that might touch the infant and make sure the surfaces do not get too hot.
Test to determine when the incubator’s temperature stabilizes, and that it stays at the temperature setting for at least an hour. Verify the temperature is same throughout the compartment. Check the accuracy of the temperature indicator, and make sure the temperature control sets the temperature to the correct value.
Measure the skin temperature sensor with a calibrated heater assembly. When testing radiant warmers, make sure the temperature distribution is accurate—meaning that the average temperature of the mid-point is the same as the average of the other test points. And finally, test to ensure the temperature control is the actual temperature sensed by the skin temperature probe.
Humidity: Relative humidity is important for respiratory care and air temperature and must be monitored to minimize heat and water loss. Check the accuracy of the relative humidity. The incubator value should be ±10 % of what the tester indicates.
Airflow: High air velocities increase evaporative water loss and heat loss of the patient. Measure the air velocity inside the incubator compartment, and ensure it is ≤ to 0.35 m/s at each location. Refer to Figure 2 for probe placement.
Sound: Infants have incredibly sensitive ears. Test to determine if the sound level inside the incubator compartment is below 60 dBA (decibels adjusted). Inside and outside alarm levels should be measured to ensure they are within a safe range, yet still audible over background noise.
Oxygen/weighing scale: Most incubators and radiant warmers double as weighing scales since moving tiny patients can be both difficult for the caregiver, and detrimental to the patient’s health. Measure the scale using a series of calibrated weights (same placement as Figure 2) to ensure it is accurate. Check the oxygen concentration to validate it is within the acceptable range, and that the visual and auditory alarms are appropriately activated.
There is a learning curve when using test instruments, especially those that are new or used infrequently. One of the best ways to shorten this learning curve is to standardize the procedure. Test standardization helps ensure all testing is completed in a consistent sequence, accurately recorded and archived, and meets regulatory requirements.
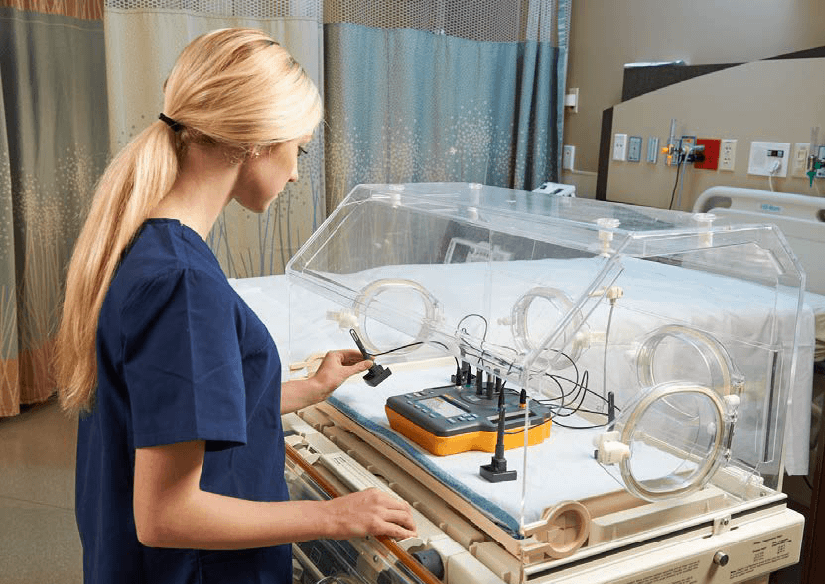
INCU II Incubator/Radiant Warmer Analyzer being used to test an infant incubator
Test automation can help reduce test time, and even increase efficiency. Testing each parameter one-by-one, using individual handheld tools can take a very large amount of time. However, when using an analyzer with built-in test automation—such as the Fluke Biomedical INCU II—test time can be dramatically reduced. According to Medical Equipment Quality Assurance: Inspection Program Development and Procedures by J. Tobey Clark, the INCU II Incubator/Radiant Warmer Analyzer can completely test an incubator in 120 minutes, and test a radiant warmer in 35 minutes. However, once an incubator has warmed up, the general testing feature of the INCU II can complete a test within just 15 minutes. The onboard automation of the INCU II will automatically run through the tests in both standard and customizable test templates. Technicians simply need to set up the device and start testing, and they are free to perform other work.
Other benefits of using test automation to validate the performance and safety of incubators and radiant warmers is data traceability, simplified data extraction for reporting, and reduced human error.
The purpose of testing and producing test results is to have a continuous stream of data showing all changes in the performance and safety of the incubator and radiant warmer year-over-year.
Long-term trending of this statistically relevant information provides the basis for predictive maintenance (i.e., when the next repair is most likely to happen) so the parts (especially long-lead time and costly parts) can be ordered and received just-in-time for the repair event. This saves money and increases the amount of time the medical device is available for use. In regard to incubators, a longer stay for the patient is both costly for the family and the hospital. Ensuring an incubator is operating properly and can be repaired in a timely fashion is important for all parties involved.
The best place to archive test result information is in a database or Computerized Maintenance Management System (CMMS). In contrast, archiving paper in filing cabinets rarely results in anyone capturing or understanding the long-term implications of failures.

Pucks are used with the INCU II Incubator/Radiant Warmer to verify the performance of a radiant warmer
The INCU II Incubator/Radiant Warmer Analyzer is the best-in-class, all-in-one tester for preventative maintenance and testing of incubators, transport incubators, and radiant warmers. The INCU II allows users to streamline their workflow and increase productivity with the following features:

Ashton Solecki, Product Marketing Manager for the Neonatal Test product line at Fluke Biomedical
Ashton is a published international researcher, scholar, and holds degrees in Cell Biology and Neuroscience, Biomedical Science, and Honors from Montana State University.
To learn more about the INCU II Incubator/Radiant Warmer Analyzer and other neonatal test equipment from Fluke Biomedical, visit www.flukebiomedical.com/INCU
Fluke Biomedical
6045 Cochran Road
Cleveland, OH 44139-3303 U.S.A.
For more information, contact us at:
(800) 850-4608 or Fax (440) 349-2307
Email: sales@flukebiomedical.com
Web access: www.flukebiomedical.com
