

Electrosurgical units (ESU) use a high-frequency electrical current to cut tissue and control bleeding by causing coagulation. Tissue resistance to the high-density current causes a heating effect which results in tissue destruction. Electrical current is delivered and received through cables and electrodes. The electrodes may be activated by either a hand piece switch or a footswitch. The ESU may use a monopolar or a bipolar mode.
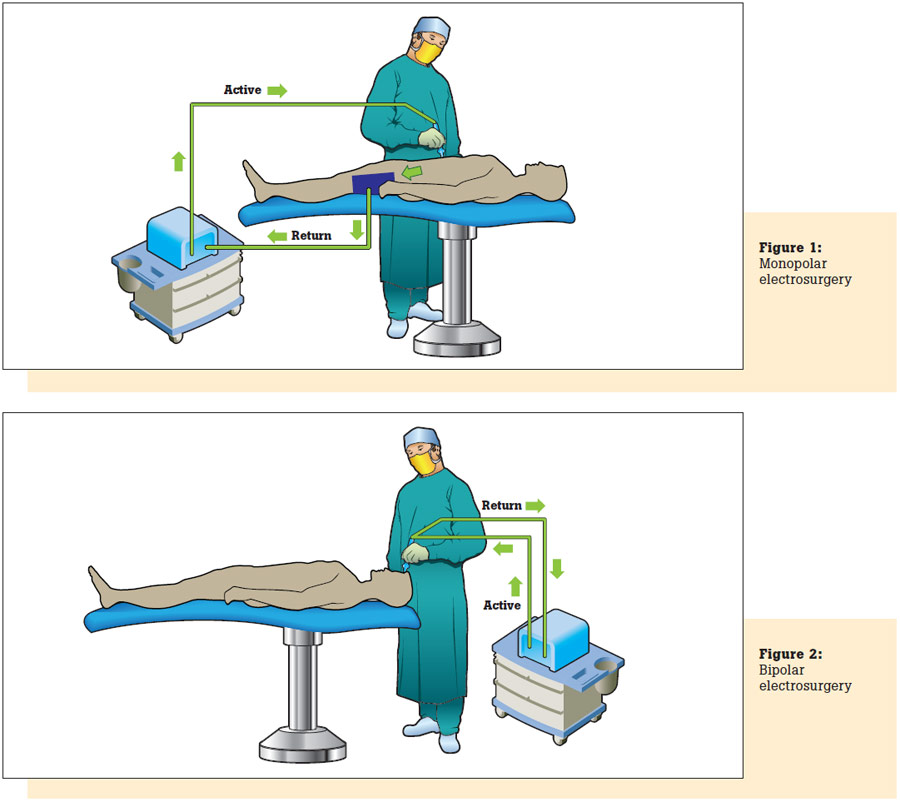
In monopolar mode, electrical current is delivered to the patient via an active cable and electrode. As shown in Figure 1, current returns to the unit through a return electrode pad or plate to disperse the return current, thus preventing focused heat which can cause burns.
In bipolar mode, two electrodes, typically the tips of a pair of forceps or scissors, serve as the equivalent of the active and dispersive leads in the monopolar mode. See Figure 2.
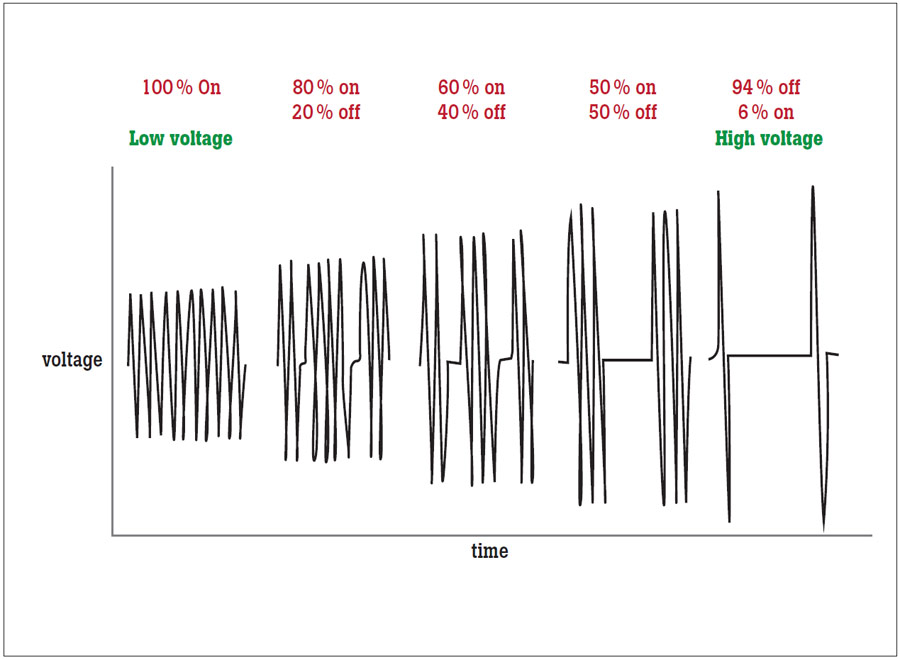
There are two types of cut modes: blended cut and pure cut. Pure cut is typically used for dissection only. In pure cut mode, the surgeon achieves a cut that is very similar to an incision produced by a medical scalpel. The cut is narrow, deep and the surgeon has little or no control over bleeding. As shown in Figure 3, this effect is obtained by high frequency and low voltage.
In blended cut mode, the surgeon achieves a much wider incision by heating up the tissue and letting it cool. This is achieved by lower frequency and higher voltage than pure cut.
Coagulation is performed by using high voltage and low frequency. In COAG mode, heat is incapable of explosive vaporization, therefore, resulting in a thermal coagulum, also known as a clot. In COAG mode, the surgeon has more control over bleeding because the tissue is allowed more time to cauterize in between contact.
Manufacture recommended test procedures should always be followed. Refer to the service manual for performance inspection tasks specific to the device. These service manuals typically recommend an inspection frequency. Complete the performance inspection per manufacturer’s procedure.
If the service manual and inspection procedure from the manufacturer is not available, the frequency of inspection must still be determined. One method for determining how often a medical device should be tested is a risk-based method used by the University of VT Biomedical Engineering Department. As shown in the table below, this method is described in Medical Equipment Quality Assurance: Inspection Program Development and Procedures by J. Tobey Clark. This method recommends a bi-annual (every six months) test frequency for electrosurgical devices.
Additionally, most major electrosurgical device manufacturers recommend semi-annual preventive maintenance testing to ensure the performance of the unit.
If the service manual and inspection procedure from the manufacturer is not available, it is still the responsibility of the medical facility to choose and standardize on a test procedure. It is important that the electrosurgery generator functionality be quantitatively evaluated by comparing it to the manufacturer’s specifications, or the requirements in the applicable medical device standard. When the medical device manufacturer’s specifications are not known, the IEC standard requirements are a reasonable substitute. Once the inspection criteria have been agreed, no changes should be made without a rationale statement describing why a change was required, what the change is, and how this change was validated.
| Criteria - choose 1 rating from each category | Weight | Score |
| Clinical function | ||
| No patient contact | 1 | |
| Device may make contact with patient but function is non-critical | 2 | |
| Device is used for patient diagnosis, or direct monitoring | 3 | |
| Device is used to deliver direct treatment to the patient | 4 | 4 |
| Device is used for a life support | 5 | |
| Physical risk | ||
| Device poses no appreciable risk due to failure | 1 | |
| Device failure will result in low risk | 2 | |
| Device failure will result in inappropriate therapy, misdiagnosis, or loss of monitoring | 3 | |
| Device failure could result in severe injury to, or death of, patient or user | 4 | 4 |
| Problem avoidance probability | ||
| Maintenance or inspection would not impact reliability of the device | 1 | |
| Common device failure modes are unpredictable or not very predictable | 2 | 2 |
| While common device failure modes are not very predictable, device history indicates that TSP testing frequently detects problems | 3 | |
| Common device failure is predictable and can be avoided by preventive maintenance | 4 | |
| Specific regulatory or manufacturers requirements dictate preventive maintenance or testing | 5 | |
| Incident history | ||
| No significant history | 1 | |
| A significant history of incidents exist | 2 | 2 |
| Manufacturers/regulatory requirements for specific schedules | ||
| No requirements | 1 | 1 |
| There are requirements for testing independent of a numerical rating system | 2 | |
| Total Score: | 13 | |
| Assignment: 0.0x 0.5x 1x 2x 3x 4x (times per year tested) | 2 | |
Most manufacturer performance inspection procedures require electrical safety tests including ground wire resistance and chassis leakage. Keep an electrical safety analyzer close by to complete the electrical safety portion of the performance inspection easily.
Additionally, a medical oscilloscope can be used to show the actual wave shape of the device under test (DUT). This waveform output can be compared to the DUT’s service manual. Please see two possible solutions below.
Keep all test leads and interconnecting leads as short as possible and do not cross or coil measurement leads. Radio frequency energy behaves differently than low frequency energy. It radiates and induces electrical current flow in addition to any conductive current flow through test leads that cross and coiled leads. When leads are too long, they act more like an antenna than test leads.

Active electrodes present many dangers. Do not touch the active electrode or return pad/plate of the ESU while it is activated in either cut or coagulation mode. Turn off the ESU before adjusting or removing connections.
Additionally, be aware of other flammable hazards including alcohol, oxygen and moisture.
Power distribution/output tests: Power distribution/output tests measure the power output properties of the ESU and supply output current (A), power (W), peak-to-peak voltage (V), and crest factor values. The power distribution test evaluates the output across multiple loads to
determine how well the impedance-sensing-circuits of new generation electrosurgery generators automatically adjust the output of the ESU so that it is not reduced by the presented load.
High Frequency (RF) leakage current tests: The RF leakage current in electrosurgical units is a critical parameter to measure because it may cause accidental burns in patients. The particular standard for electrosurgical units, IEC 60601-2-2, indicates the maximum RF leakage levels and defines the elements and their layout to do these measurements.
RECM tests: The RECM (return electrode current monitor) is the “watch-dog” that alarms (both audibly and visually) and prevents the electrosurgery generator from energizing when the high limit threshold for current flowing through the return electrode plate or pad has been exceeded.
Inert gas flow and pressure parameters: In some electrosurgery generators a special option allows an inert gas envelope to be produced encasing the surgery site so that oxygen is eliminated at that specific spot. Oxygen causes charring of the tissue at the site of the surgery. Eliminating oxygen prevents this charring and produces cleaner, more precise incisions. These more precise wounds heal faster, with less opportunity for infection of the tissue. Test the gas flow and pressure for such inert gas outputs.
One of the best ways to shorten learning curves for infrequently used test instruments and new or infrequently scheduled testing is to standardize the procedure.
Test standardization helps ensure all testing is completed in a consistent sequence, recorded and archived accurately, and meets regulatory requirements.
Test automation can also drastically reduce test time. According to Medical Equipment Quality Assurance: Inspection Program Development and Procedures by J. Tobey Clark, the average test time for most electrosurgical unit tests is 35 minutes. When coupled with the QA-ES III Electrosurgery Analyzer, Ansur Test Automation Software can reduce average test time to under 15 minutes.
Other benefits of using test automation to test electrosurgical units include: easy data traceability, simplified data extraction for reporting, and reduced human error.
It is critical that data gathered is recorded accurately. Data can be recorded manually or electronically. Manual recording could be documenting on paper or typing into an electronic source such as a PC. Some issues associated with documenting manually include misplaced records, legibility, skipped tests, and recording errors. This method is time consuming as well. To ensure data integrity, record electronically. Consider an analyzer that has the ability to store data. The purpose of testing and producing test results is to have a continuing stream of data showing all changes in the performance and safety of the electrosurgery generator year over year.
Long term trending of this statistically relevant information provides the basis for predictive maintenance (i.e., when the next repair is most likely to happen) so that parts (especially long-lead time and costly parts) can be ordered and received just-in-time for the repair event. This saves money and increases the amount of time the medical device is available for use.
The best place to archive test result information is in a database/CMMS (computerized maintenance management system). Archiving paper into filing cabinets rarely results in anyone capturing or understanding the long-term implications of failures.
Use the following criteria when selecting an analyzer for electrosurgical device testing:

Depend on the QA-ES III Electrosurgical Analyzer for complete ESU preventive maintenance, and safety testing of all critical functions. Collect all measurements including vessel sealing, contact quality monitor (CQM), high frequency (HF) leakage and output power distribution in single or continuous mode.
The QA-ES III has all hardware and software needed for complete testing, so you don’t need to carry additional accessories or cables. With all-in-one features, data storage, wireless functionality*, the QA-ES III is one of the most user-friendly electrosurgical analyzers on the market today. Additionally, Ansur test automation software automates testing and allows users to create and automatically run tests, capture data, and produce easy-to-read reports.
*Wireless capabilities not available in all countries. Ask your sales representative for more details.
The Product is a precision instrument for use in performing tests on high-frequency electrosurgical units in accordance with national and international standards. It is for use by trained service technicians. The Product will be used in hospitals, clinical engineering departments, independent service organizations, and at ESU OEMs. The Product will not be used in patient rooms while a patient is present.
Fluke Biomedical
6045 Cochran Road
Cleveland, OH 44139-3303 U.S.A.
For more information, contact us at:
(800) 850-4608 or Fax (440) 349-2307
Email: sales@flukebiomedical.com
Web access: www.flukebiomedical.com
